Detailed chemical kinetics models for fuels are typically comprised of hundreds or even thousands of individual chemical reactions describing the interactions (i.e., collisions and rearrangement of atoms) between each species and radicals involved in the combustion of the fuel(s) of interest. The aim of detailed kinetics models is to predict as accurately as possible the reaction progress of a given fuel over a wide variety of conditions in terms of pressures, temperatures, concentrations, and equivalence ratios, so the models can then be used with confidence during the design phase of various applications such as rockets, internal combustion engines, and gas turbines.
To accurately predict the combustion progress of fuels, their detailed chemical kinetics models need to be validated. The experimental results produced in our laboratory, from global kinetics data involving many reactions (ignition delay time, laminar flame speed) to speciation and rate coefficient measurements are used to help develop and validate these models.
Over the past few years, our group has produced a large body of experimental results, which have been used to develop detailed kinetics models in collaboration with other groups (Hydrogen, syngas, natural gas, propene, isobutene, pentene) or internally for the combustion of specific fuels (ammonia, nitromethane). A large part of our activities in this area is also devoted to the study of interactions between fuels such as H2 and CH4 and pollutants and impurities (H2S, NO2, N2O) during combustion.
Our group is also active in the field of fire suppressants for safety applications. A large number of candidate fire suppressants have been studied, from halons such as CF3Br, CF3I, and C3HF7 to phosphorus-containing agents such as dimethyl methylphosphonate (C3H9O3P). Based on the experimental results produced in our group, their detailed kinetics models have been improved, and a unique, detailed kinetics mechanism for CF2BrCl was developed. The second figure below shows a comparison of the CF2BrCl kinetics model with experimental ignition delay time data from our group.
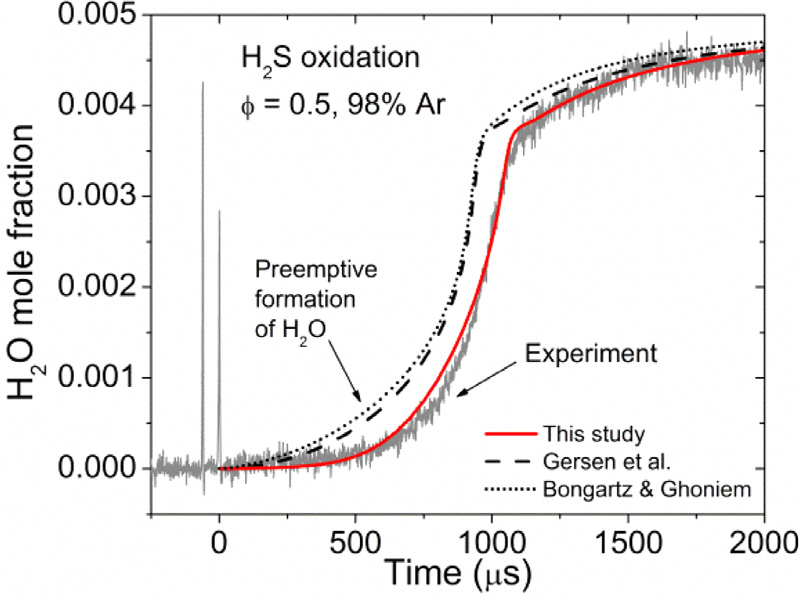
Example of a water mole fraction time history behind a reflected shock wave and comparison with models from our group and from the literature for a mixture of H2S diluted in 98% Ar at around 1 atm.
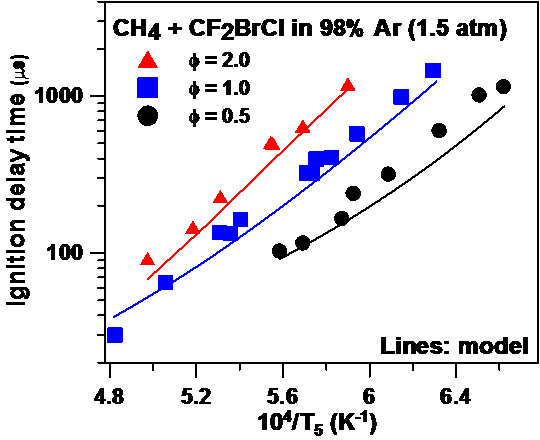
Comparison between experiments and model for a mixture of CH4 diluted in 98% Ar at around 1.5 atm, with an addition of CF2BrCl at 10% of the CH4 concentration.