The highly monochromatic nature of narrow-linewidth lasers means they can be anywhere from 100 to 10,000 times narrower than the UV, visible, or IR spectral features of the absorbing species. Thus, by fixing the wavelength of the laser at a wavelength where the absorbing species will absorb strongly, the absorption coefficient can be calculated at that specific wavelength. By tracking the attenuation of the laser over the course of a combustion event, the time-resolved concentration of the absorbing species can be measured and then utilized for the refinement of chemical kinetics mechanisms. Shown in the figure below is an example of an experimental H2O time history, from which it was possible to suggest a factor-of-30 decrease in the rate constant of the reaction H2+N2O ↔ H2O+N2.
Fixed-wavelength diagnostics can also be applied to non-combusting environments. For example, such diagnostics can be utilized to measure the initial concentration of a species in a mixture to ensure that complications such as condensation are not an issue. Fixed-wavelength diagnostics have also been employed to ascertain the level of particulate matter (e.g., aluminum nanoparticles) present in heterogenous mixtures.
Species that have been measured using fixed-wavelength diagnostics either at Texas A&M or in collaboration with others, namely The Aerospace Corporation, include CO, CO2, H2O, CH, SiH2, OH, H2O2, and various hydrocarbons.
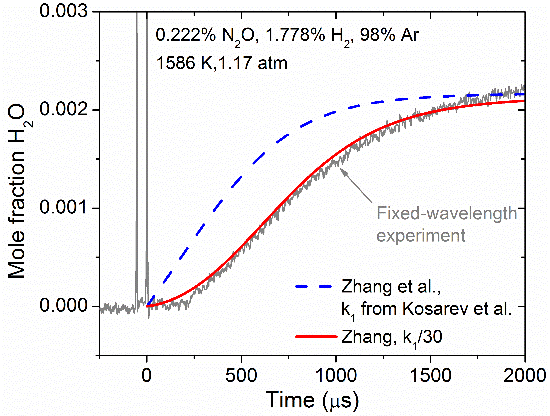
Experimental H2O time history behind a reflected shock wave in a mixture of H2 and N2O. Adapted from [1].
References:
[1] C. R. Mulvihill, O. Mathieu, and E. L. Petersen, “The Unimportance of the Reaction H2 + N2O = H2O + N2: A Shock-Tube Study Using H2O Time Histories and Ignition Delay Times,” Combustion and Flame, Vol. 196, 2018, pp. 478-486.