One of the key measurements used to validate chemical kinetics mechanisms is the laminar flame speed. Ideally, the laminar flame speed, SL, is the speed at which an adiabatic, one-dimensional flame propagates through a doubly infinite fuel/oxidizer mixture. This ideal case is shown in the figure below. There are several different methods that can be used to approximate this ideal condition, each with different limitations. However, they all require some extrapolations to obtain the ideal flame speed. At a practical level, the flame speed dictates whether a flame in a moving flow will remain stable, and it has been shown that turbulent flame speeds can be correlated with SL. However, few reliable SL data exist at elevated pressures, and we have one of the few facilities from which high-pressure laminar flame speeds can be obtained.
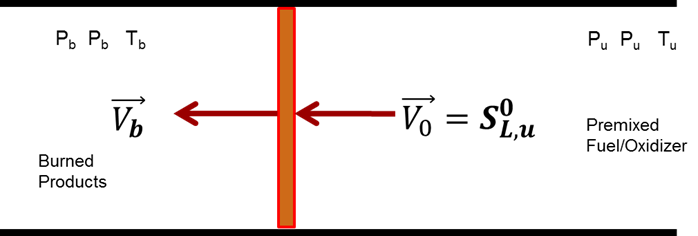
Ideal condition of an adiabatic, one-dimensional flame.
For both laminar and turbulent flame speed experiments in our laboratory, fuel-air mixtures are prepared in one of two stainless-steel, constant-volume vessels. This mixture is typically prepared using the partial pressure method. The premixed fuel-air mixture is then centrally ignited, and the flame propagates through the fuel-oxidizer mixture.
More details on fundamental flame propagation research in our group is found in: