In our laboratory, the spherical flame method is used to measure the laminar flame speed. This technique directly measures the growing radius of the spherical flame over time using a schlieren imaging technique. This result is then extrapolated using the appropriate non-linear methods to the ideal, un-stretched flame speed. This technique also requires the removal of ignition effects near the beginning which increase the flame speed, and confinement effects as the flame approaches the wall which decrease the flame speed. Equilibrium chemistry is then used to calculate the flame speed of the unburned gases. One of the benefits of this technique is that it allows for high-pressure initial conditions. The optical access and visualization of the images also allows us to verify that the flame stays laminar and does not become unstable and turbulent.
Flame speed experiments are typically conducted over a range of equivalence ratios from lean to rich. For typical hydrocarbons, the peak flame speed is normally around φ = 1.1. For fuels with a high hydrogen content (such as syngas) the peak flame speed is usually closer to φ = 2.0. Sample results are shown in the figure below, in this case for propene (C3H6) in air at 1 atm and 295 K.
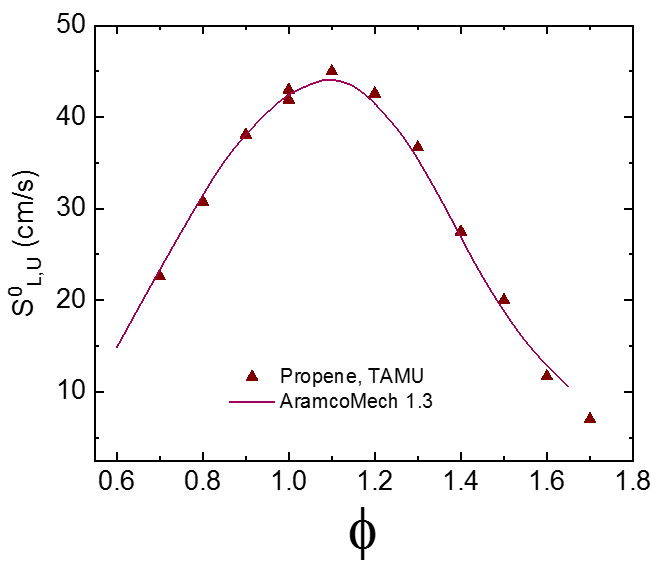
Sample laminar flame speed plot, showing data for propene (C3H6) flames at 295 K and 1 atm.