In a typical combustion event, an energy source such as a spark or a shock wave is used to add energy to reactants and initiate the combustion process. During this localized energy addition, the various atoms and molecules in the mixture take on that energy by absorbing it inelastically within the structure of their atomic and/or molecular bonds, or they absorb it elastically causing their velocity to increase (somewhat like billiard balls do on a pool table). Prior to energy addition, the components of the mixture are interacting, but not chemically. Because of this absorption of energy, however, the reactants in the mixture become prone to interact chemically, which releases many different kinds of radiation due to atomic and molecular energy-level transitions. This radiation emission during combustion is a familiar sight to anyone who has lit a match, used a Bunsen burner, or sat around a campfire. Such combustion events are giving off radiation in the form of light and heat, and one type of light emission is known as chemiluminescence, or light emission due to the presence of chemical reactions.
Since a global combustion event is not characterized by merely a single chemical reaction but rather by several reactions, many different radicals and compounds are present at any instant during the combustion process. Additionally, because of the variety of species present during combustion, myriad energy transitions are also occurring and emitting a spectrum of wavelengths. These emitted wavelengths are not random, however, and each atomic or molecular quantum-mechanic energy transition that occurs is accompanied by the emission of a photon at a specific wavelength of light that corresponds to the energy difference between the excited and unexcited states of the molecule(s)/atom(s) emitting that wavelength. Thus, chemiluminescence can be observed over a large spectrum of wavelengths, but it can also be used to detect precise wavelengths for energy transitions of specific species.
In general, light emission indicates that a combustion event is occurring, and the progress of the combustion event can be tracked by measuring the emission of specific wavelengths of light. Furthermore, the observation of specific wavelengths indicates the presence of specific, critical radicals, the detection of which aids in tracking various characteristics of global chemical reaction progress during a combustion event (or even to the progress of a single reaction). For example, ignition can be detected when certain wavelengths are emitted during the transition of several key radicals from their electronically excited states to an unexcited state. Some of these key radicals include the excited-state species of the OH, CH, C2 and CO2 molecules; OH*, CH*, C2*, and CO2*, respectively. By measuring the location and intensity of chemiluminescence due to the relaxation of these species (thus releasing a photon), one can obtain spatial information on where the main reaction zones are present in a laboratory flame burner or practical combustor. Chemical kinetics information can also be obtained in an unsteady combustion event, such as those in a shock tube or a flame bomb, by measuring chemiluminescence emission as a function of time during the course of the event. To make such measurements quantitative and to also relate the measurement of the aforementioned excited-state species to their ground-state counterparts, knowledge of the chemical kinetics of the chemiluminescence process is required.
Using our shock-tube and flame speed capabilities at Texas A&M University, we have been making measurements of the rate coefficients of key chemiluminescence reactions. For example, we have previously measured the rates of and at high temperatures. When in the presence of silicon (such as from silane-related experiments), the chemiluminescence of OH* required the addition of a new reaction to account for the observed OH* time histories in hydrocarbon-silane and hydrogen-silane oxidation experiments. We also commonly employ chemiluminescence as a diagnostic in shock-tube ignition and oxidation experiments, and detailed knowledge of the chemical kinetics can be used to check the predictive capabilities of chemical kinetics models. Two papers we have previously written on the kinetics of OH* chemiluminescence are given by Hall and Petersen [1] and Hall and coworkers [2].
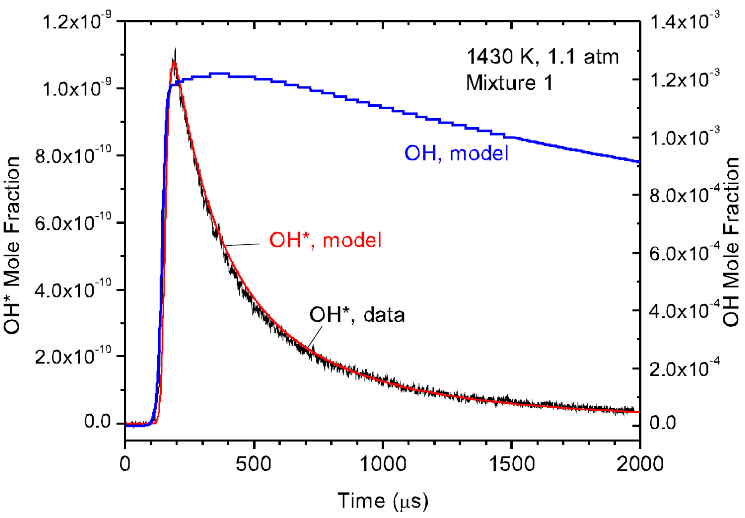
Measured OH* time history in comparison with new model prediction for a dilute H2/O2 mixture in Ar; alsi shown is OH (calculated) as reference
References:
[1] J. M. Hall and E. L. Petersen, ”An optimized kinetics model for OH chemiluminescence at high temperatures and atmospheric pressures,” International Journal of Chemical Kinetics, Vol. 38, 2006, pp. 714-724.
[2] J. M. Hall, S. Reehal, and E. L. Petersen, “Kinetics of OH chemiluminescence in the presence of silicon,” Chemical Physics Letters, Vol. 425, 2006, pp. 229-233.