Liquid monopropellants are utilized for in‐space reaction control systems (RCS) due to their modest performance, design simplicity, restart capability, and high repeatability. However, the most-utilized liquid monopropellant, hydrazine, only offers modest specific impulse, is expensive to store and field, and is extremely toxic. Accordingly, research interest in a new ‘green’ replacement for these systems has been sparked over the last few decades. We have been involved in evaluating the baseline combustion behavior, materials compatibility, and safety properties of some potential replacements, such as the hydroxyl ammonium nitrate (HAN)‐based propellant developed by the United States Air Force (AF‐M315E) and the ammonium dinitramide (ADN)‐based propellant developed in Switzerland (LMP‐103S). Ballistic experiments take place in a constant‐volume burner with high‐speed video, pressure, and spectroscopic diagnostics. An example of nitromethane and 82.4% aqueous HAN combustion is shown below. Evaluation of propellant safety properties has primarily been comprised of thermal decomposition (DSC, TGA, DTA) and thermal cook‐off experiments.
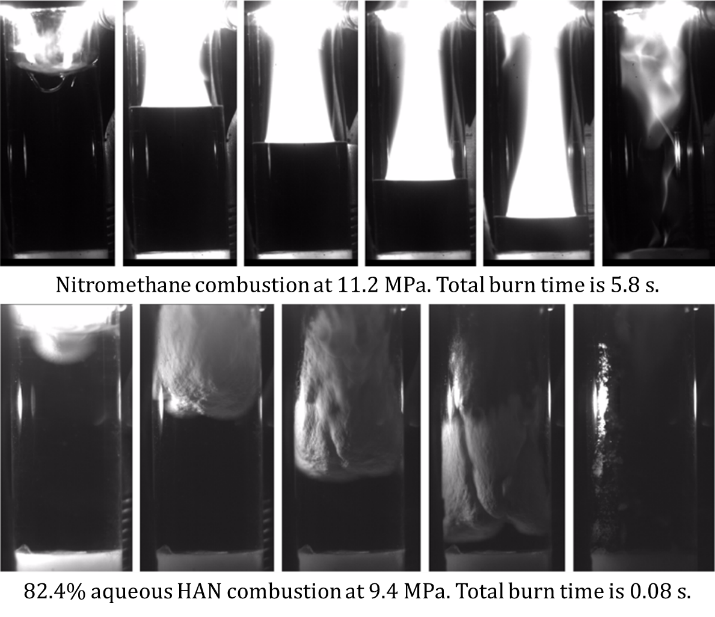
We have also focused on improving and tailoring the ballistic performance of existing liquid monopropellant systems through the evaluation of commercially available additives and the development of novel additives. For example, these studies include commercially available metal‐oxides which are capable of independently altering the liquid‐ or gas‐phase combustion rate of some liquid monopropellants (see the below figure) and pure metals which can alter the combustion rate of propellant systems, as well as serve as a dense fuel source. Novel metal‐based additives have been developed in conjunction with our collaborators at the University of Central Florida and Helicon Chemical, and are currently being evaluated for various liquid propellant systems.