During a shock-tube experiment, the test gas is nearly instantaneously compressed to a known pressure and temperature. This step change in conditions makes shock tubes ideal devices to study the chemical interaction of species at elevated temperatures. In our laboratory, we utilize lasers to collect measurements of several species of interest during the test time.
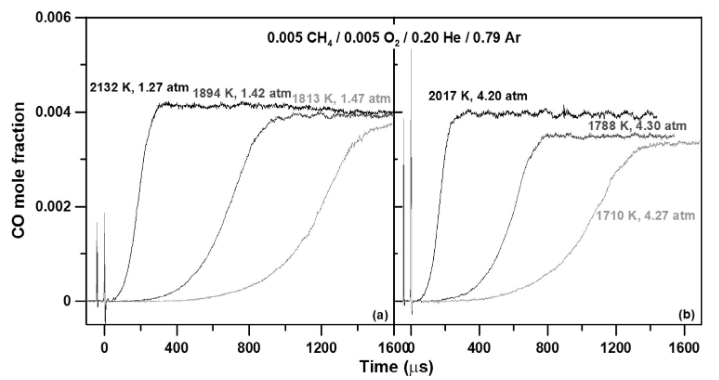
CO concentration measurements during different shock-tube experiments [1].
The fact that different species absorb light at different frequencies allows us to tune our laser’s frequency to match the species of interest. In the past years, we have been active in performing experiments to measure several species concentrations such as CO, CO2, H2O, CH, SiH2, OH, H2O2, and various hydrocarbons. These measurements can be extremely valuable to assess the performance of detailed chemical kinetics mechanisms. For example, shown in the above figure is a recent study where CO formation was measured in a methane mixture at different conditions. These measurements were then compared with detailed chemical kinetics mechanisms to assess their performance, examples of which are shown in the below figure. For more details on speciation measurements, see the direct laser absorption section.
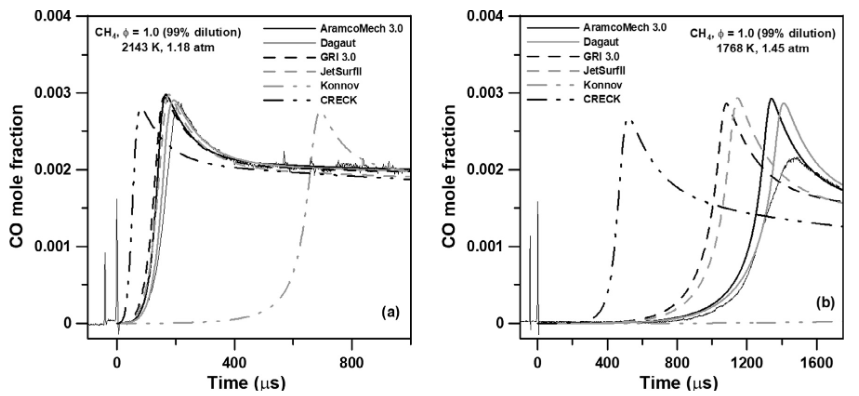
Comparison of CO concentration measurements with the predictions from different detailed chemical kinetics models [1].
References
[1] O. Mathieu, C.R. Mulvihill, and E.L. Petersen, Assessment of Modern Detailed Kinetics Mechanisms to Predict CO Formation from Methane Combustion Using Shock-Tube Laser-Absorption Measurements, Fuel, Vol. 236, 2019, pp. 1164–1180.